复杂血管内装置脉动耐久性试验
Pulsatile durability testing is performed to evaluate an intravascular device’s resistance to fatigue failure.
Intravascular devices are typically placed within straight mock arteries for testing, mounted onto a stent/graft tester and subjected to cyclical pressure pulses. The pulses create a dynamic radial strain in the mock artery that simulates in vivo strain levels that occur due to the expansion and contraction of the surrounding vessel.
复杂的血管内装置
虽然许多设备设计使用直容器进行测试, complex device geometries occasionally require the development of custom mock arteries to simulate target deployment sites. 如果引入定制的模拟容器, additional validation and feasibility testing are required.
Examples of such devices include left atrial appendage 遮光板 to prevent strokes in non-valvular atrial fibrillation patients, 神经血管栓塞线圈治疗复杂脑动脉瘤, endovascular aneurysm repair devices of abdominal aortic aneurysms, 以及动静脉瘘的设备.
定制模拟动脉测试设备
MDT (now im体育APP) designed the ElectroForce 9100 Series of Stent/Graft testers specifically for pulsatile durability testing. Its dual motor design is perfectly suited for custom applications as it allows tweaking of the amp of one of the motors to get more uniform strains and improved strain distribution across the length of larger vessels, 包括腹主动脉瘤(AAA)装置. 单电机支架测试仪缺乏这种能力.
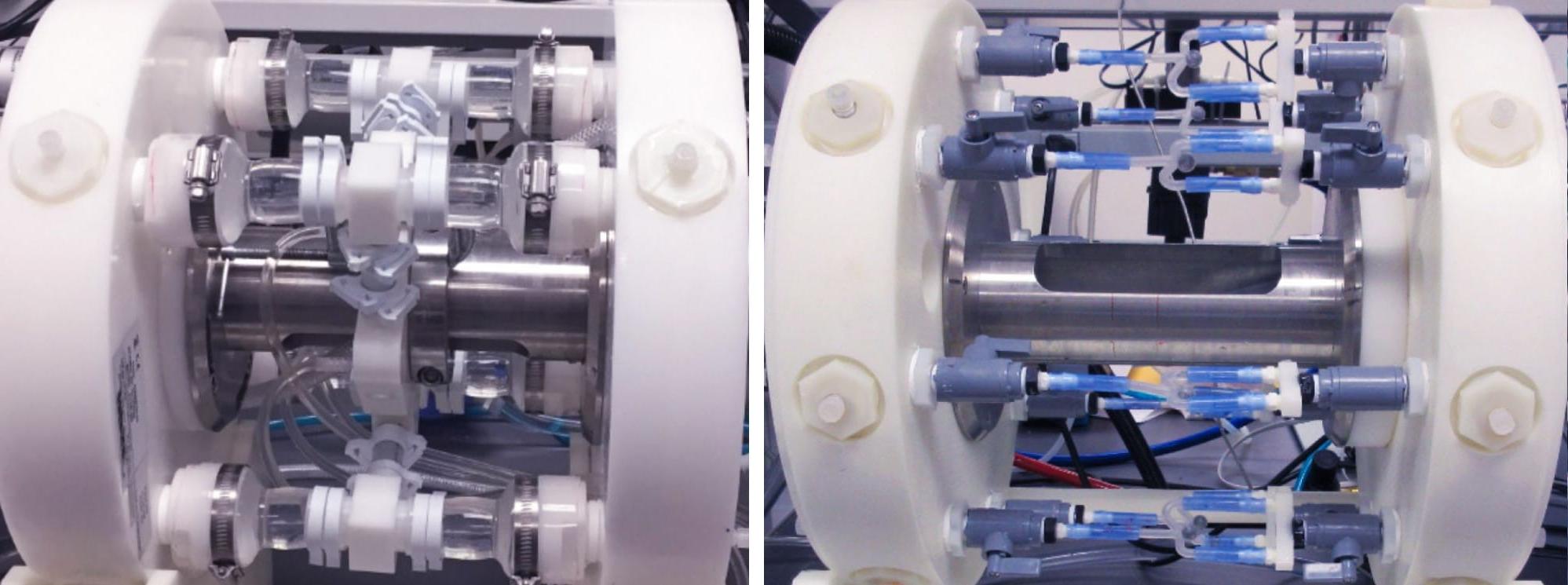
The ElectroForce 9100 instrument features 4 to 12 mock arteries mounted between a pair of manifolds and linear-motor-driven pumps. The computer-controlled system delivers radial strain to intravascular devices under controlled temperature conditions, and a laser micrometer measures radial strain at various positions along the length of each device to ensure that desired strain levels are met throughout testing.
The manifolds are modular and allow mounting customization of mock vessels and diameters to match each application such as the neurovascular coil testing shown below (left image).
The setup below (right image) was used for pulsatile fatigue testing of 8 left atrial appendage 遮光板 (two per station) at 40 Hz and 37±2°C in isotonic saline solution. 测试 was performed per ASTM F2477-07 (2013) and ISO 25539-1:2003/Amd1:2005 (now incorporated into ISO 25539-1:2017). 模拟硅胶容器有两个部分, a smaller diameter section and a step-up larger diameter section to accommodate the occluder geometry.
血管内装置脉冲耐久性试验步骤
The table below outlines the main steps of a standard experimental protocol. 如果引入定制的模拟容器, additional validation and occasionally feasibility testing take place to ensure that devices are reaching target strain levels.
- 检查试验船并记录观察结果
- 对脉动测试系统进行容器调节
- 将设备部署到测试容器中
- 检查设备并记录观察结果
- 将部署的容器安装到脉动测试系统上
- Fill pulsatile test system with saline or distilled water and control the temperature to 37°C
- Begin cycling and adjust the mean pressure to achieve the vessel diameter target
- 调节体积位移或ΔP以达到应变目标
- 一旦达到目标就开始疲劳试验
- 每天监控测试目标的一致性和稳定性
- Inspect devices at interim timepoints in accordance with the test protocol
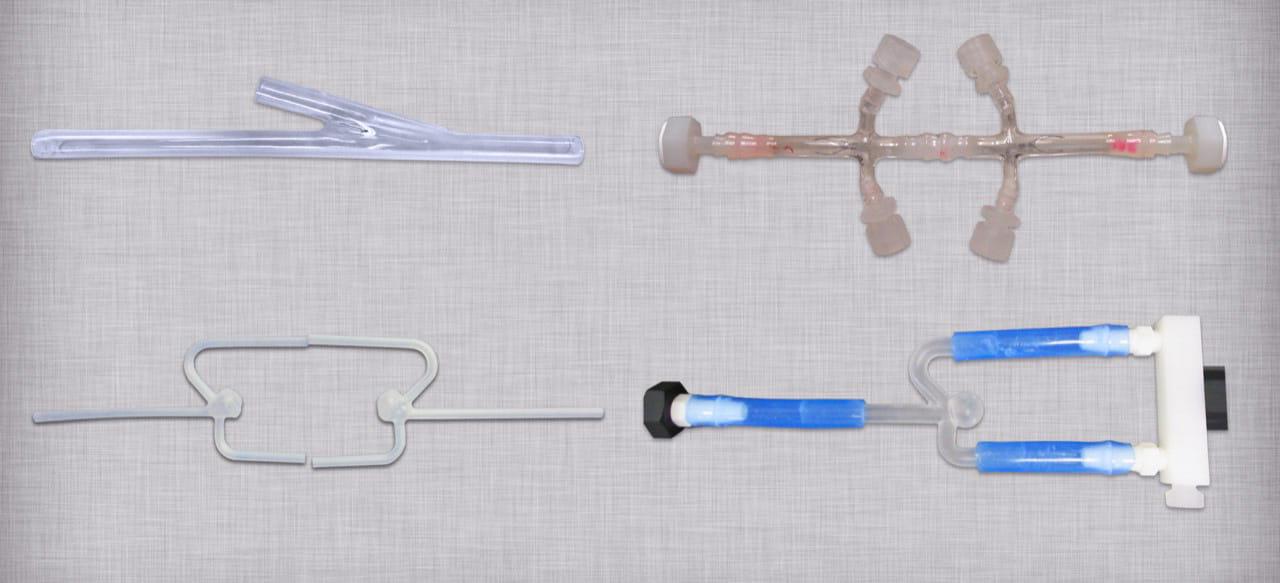
设计右侧模拟动脉
Design requirements for the custom mock vessels are typically obtained from published data in the literature and/or finite element analysis. 两个主要输出是容器的几何形状和顺应性. 模拟生理血管顺应性, these mock models are often manufactured from silicone as shown below.
验证测试关注于应变和遵从性目标. In some applications, reaching the target strain level is more important than vessel compliance. However, compliance targets are critical in physiological modeling applications.
A laser micrometer is used to measure strain validation by mapping strains along the custom mock artery and making instrument adjustments until reaching the target values. 类似的, target vessel compliance is verified by taking outer diameter measurements with the laser micrometer and converting these values to inner diameter. 校准的压力传感器测量压力水平, 柔度计算公式如下:
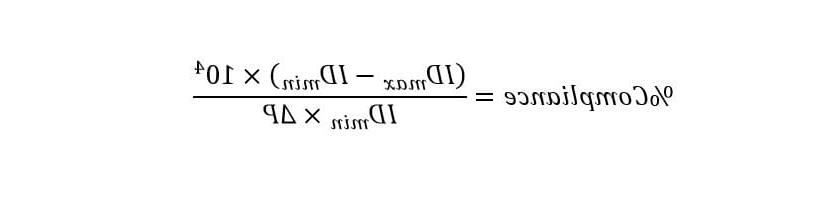
总之, complex device geometries and deployment sites often require the development of custom mock arteries. This complexity added to the test setup requires additional validation to ensure that devices are subject to the desired test conditions.
我们能为您做些什么?
Our 从事专家 excel at intravascular pulsatile durability testing and have worked with many challenging device designs and test setups over the last several decades. im体育APP 讨论我们如何帮助你的测试项目.
im体育APP Materials 技术 is ISO 17025 accredited and has one of the most expansive medical device testing scopes in the world ranging from orthopedics and cardiovascular implants testing to EMC/EMI/product safety testing, 以及生物和包装评估.
找到相关的 资源
相关服务

高速支架和移植物测试仪
Our medical device manufacturing customers are regularly challenged with short timelines to bring products to market.

心血管器械检测
im体育APP specializes in a wide range of testing for cardiovascular devices, 比如支架, 移植, 遮光板, 导管, 心脏瓣膜和起搏器导线.

医疗器械试验规程
一个协议和计划会降低你的风险, 防止混淆, 设定明确的期望, and preserve the necessary information for future reference and use.

im体育APP的位置
Learn more about our laboratories - where they are located; the unique capabilities they have and how they can help you solve your technical and commercial challenges.
